Doctors at the Spine Center are taking a leading role in the advancement of the Artificial Disc Replacement. Getting implantable devices like these from the lab to patients requires rigorous testing and approval by the Food and Drug Administration (FDA). These doctors are involved in both aspects. Dr. Paul C. McCormick, head of the Spine Center, sits on an FDA panel and Dr. Michael G. Kaiser is currently in Phase III of trials for an artificial Disc for the cervical spine.
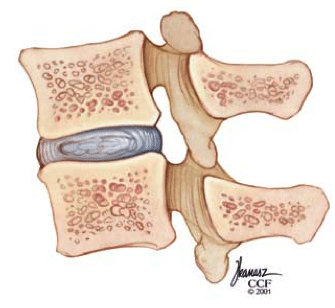
The disc in the artificial disc replacement refers to a cushiony pad between each vertebral body of the spine. These discs are made up of a fibrous outer surface and a jelly like interior and are well designed for shock absorption. As we age, however, the disc can degenerate causing its outer lining to thin or crack allowing the jelly-like center to ooze out, or herniate. This can be extremely painful, especially when it puts pressure on the large nerves nearby.
The condition is almost always treated conservatively at first with rest, medication, and physical therapy. But in advanced cases this may not be enough and removal of the disc is advised. When the problem is in the neck, the procedure that is typically done is called an anterior cervical discectomy and fusion (ACDF). Here, surgeons replace the disc with a spacer and fuse the area with bone grafts, metal plates and screws. According to the Spine Center, “This may alleviate pain but has potential disadvantages, including loss of motion and flexibility and possible further degeneration of adjacent discs.”
As an alternative, scientists have recently been developing artificial discs that can replace the degenerated ones without losing the natural movement of the neck. Because these disc-like devices can withstand movement and are secured to the bones above and below, no fusion is necessary.
Before surgical implants like these can be used widely, they have to be tested, re-tested, and then tested again. Finally, they have to pass the strict criteria of the FDA’s Medical Devices Advisory Committee. Specifically, they need to pass the subcommittee, Orthopaedic and Rehabilitation Devices Panel, a panel of experts in the field including, head of the Spine Center, Dr. Paul McCormick.
The purpose of this panel is to review and evaluate, “data concerning the safety and effectiveness of marketed and investigational orthopedic and rehabilitation devices and makes appropriate recommendations to the Commissioner of Food and Drugs.”
The very first artificial cervical disc approved by the FDA, the PRESTIGE Cervical Disc, was approved in July, 2007 and since then, a number of other models have come out. Last May, the committee conditionally approved the Bryan Cervical Disc.
Currently, Dr. Michael Kaiser from the Spine Center is conducting Phase III clinical trials for the prospective Randomized Clinical Investigation of the SECURE-C Artificial Disc. To view patient brochures explaining the clinical trial currently under way at the New York Presbyterian, Columbia Medical Center Spine Center please see here: SECURE®-C Cervical Artificial Disc
Learn more about Degenerative Spinal Disorders and Herniated Intervertebral Disc Disease